The IND application for YKST02 has been approved, and Phase I clinical trials will start soon
On April 30, 2024, YKST02, an innovative bispecific antibody developed by Excyte Biopharma Ltd, officially received IND approval from the Center for Drug Evaluation of NMPA. YKST02 targets BCMA and CD3 for the treatment of multiple myeloma.
The approval of this IND is Excyte's second bispecific antibody to transition from the lab to clinical research, demonstrating the company's steady and mature R&D efforts and laying a solid foundation for the development of subsequent pipelines.
YKST02 is a bispecific antibody with a 2+2 symmetric structure, based on the company's Fusion of IgG and scFv technology (FIST). It is a First-in-Class drug positioned as best in class.
A series of preclinical studies, including binding, activation, killing, cytokine release, in vivo pharmacodynamic model studies, and in vivo safety pharmacology studies, have fully demonstrated that the clinical safety and efficacy of the drug may be superior to Teclistamab.
Co-founder and CEO Dr. Qing'an Yuan stated, "Our company is dedicated to the development of products that possess global commercial value and superior therapeutic effects. Currently, the primary challenge in the development of CD3 bispecific antibodies for activating T cells to kill tumor cells lies in the clinical safety issue caused by severe cytokine storm (CRS). In order to address this concern, our company has developed the FIST platform, which effectively mitigates the risk of CRS through multiple approaches while also significantly enhancing the drug's half-life and yield, thus positioning it as a next-generation product. The superiority of the FIST platform has been clearly demonstrated in the Phase I clinical trial of our first drug, YK012.
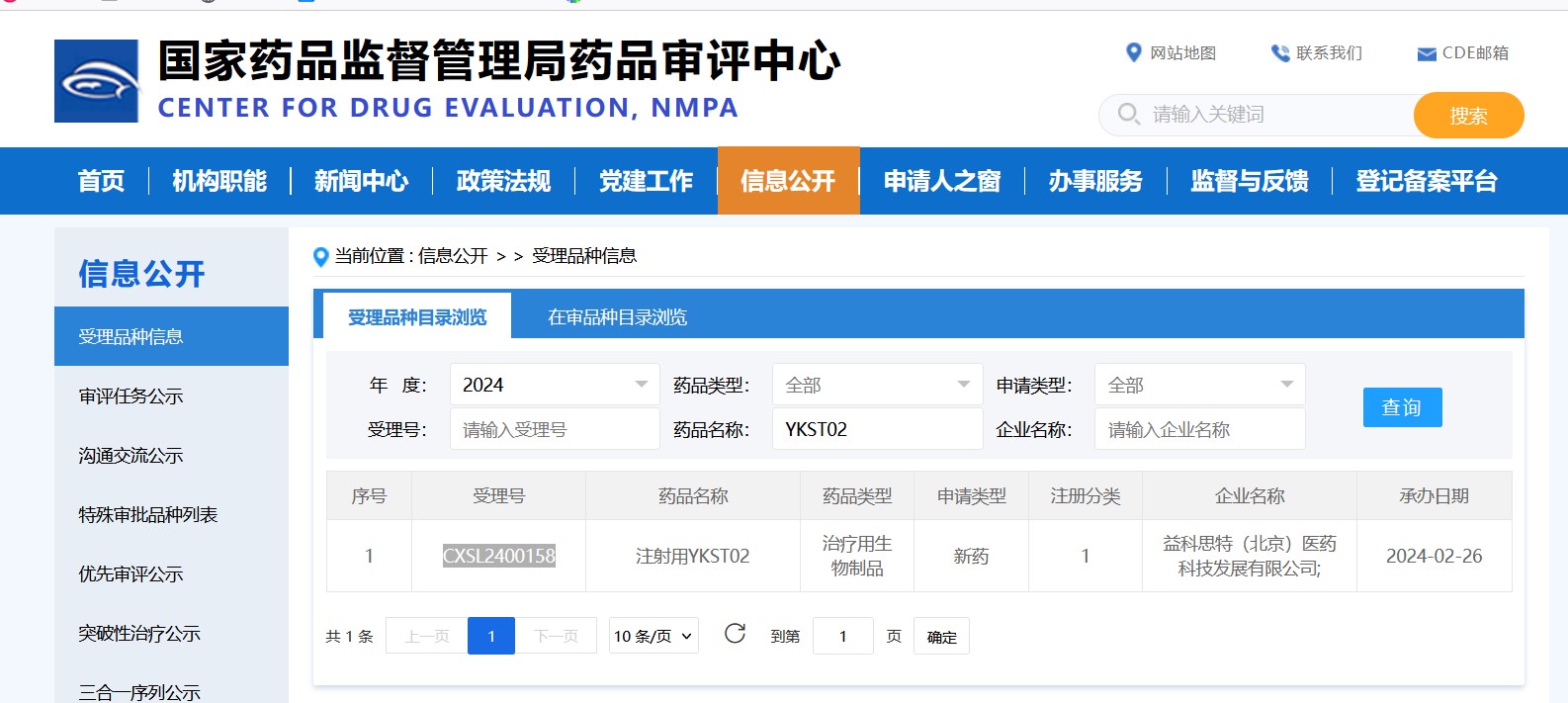
YKST02, a drug for multiple myeloma, has a mechanism of action similar to CAR-T. Preclinical data shows it is safer and more effective than Teclistamab. It was approved just two months after submission on February 26, 2024, demonstrating the strength of preclinical research and paving the way for clinical trials. The company and its partners are excited about advancing YKST02's clinical trials and exploring new indications.
Based on the FIST platform, the company is developing other urgently needed drugs. Our guiding principle is to create affordable and effective drugs for the general public, with the goal of benefiting more patients through our efforts.
Excyte was co-founded by Meng Qingwu and Yuan Qing'an, both returning from a renowned international pharmaceutical company. The company leverages the information and technology of China and the United States to develop innovative bispecific antibodies for treating blood cancers, multiple myeloma, triple-negative breast cancer, etc. Many projects demonstrate long-acting, low toxicity, high productivity, and are updated versions of international benchmark products.

